Has your child ever come to you after playing a smartphone game and asked, “How do batteries work?” So, you tell them that batteries store electricity. Then they look into your eyes and ask you, “What’s ‘lectricity?” And apart from making a noise sounding like “Derp”, you scramble to change the subject because you suddenly realize you’re not quite sure, either.
And guess what? That's OK!
To help you both get started, we’ve selected four basic and safe science projects you can do in your kitchen. While most of the materials you’ll need are probably already in your home, you will need a few important things from a home improvement or supply center.

- A Multimeter. This device measures AC and DC voltage, amperage, and resistance (ohms). A good, yet inexpensive one costs around $20 at home centers and can be very handy to have around the house.
- A Pair of Alligator Test Leads.These are wires with little clips at the ends that let you connect circuits.
- A 6-Volt Lantern Battery.These put out a good, safe level of power.
- A Red LED Light. LED stands for “Light Emitting Diode”. You’ll want one of these and NOT a red LED light bulb.
- Enameled Copper Wire.This wire is used for making coils and has a thin coating of insulation.
While you won’t be needing to solder any connections in these circuits, you will need to double check that your connections are conducting electricity through them.
PLEASE NOTE: None of these circuits or their components are designed to be plugged into household wall sockets or use household current. They are too weak for that level of power and connecting them to household current is extremely dangerous.
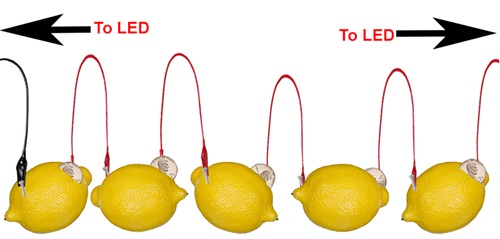
1) Lemon Power!
Building an electro-chemical battery with a lemon is THE classic science fair experiment. Although you can use almost any fruit (or potato), all you need is one galvanized nail and a single copper penny (although using a copper nail works better). Insert a zinc-coated nail (also known as “galvanized”) into the lemon through one side and insert a copper penny into the other. The zinc and the copper must be as far away from each other as possible on not touch inside the lemon.
Here's How It Works
The lemon battery works through an electro-chemical reaction when it is connected to a complete circuit. The citric acid in the lemon acts as an electrolyte, a solution that conducts electricity. The zinc nail sheds electrons as electrically charged ions into the acid (a process called “Oxidation” because the material loses electrons).
The zinc nail will have a negative charge. These electrons flow out the wire, through the circuit and re-enter the lemon through the copper penny. Here, two positively charged hydrogen atoms pick up the electrons and become an uncharged hydrogen molecule that bubbles away. The copper side will have a positive charge.
- Set your multimeter to volts DC (sometimes shown as VDC) and set it to the lowest range.
- Put the red probe tip on the penny and the black probe on the zinc nail. Hopefully, you’ll get a reading of .5 volts or more. The average lemon output is .9 volts at .00024 amps — or about .000216 watt. It takes about four or five lemons to power a single red LED (1.5 volts).
- To make this work, you’ll need to make three or four more lemon batteries and then connect them together in a series.
- That means the zinc nail of your first lemon will be connected to the LED and its copper will be connected to the zinc nail of your next lemon and then that lemon’s copper will connected to the zinc nail of the next lemon and so on.
- Do this unti the last lemon’s copper can be connected to the LED. Check your connections and the voltage output with your multimeter.
LEDs are a type of diode, meaning that they only let electricity flow one way — sort of like a valve. You have to connect your wires to it using the correct polarity: positive connects to the positive side, negative to the negative side. If you connect your LED and it doesn’t light, simply swap your connections on the LED.
What Can You Do with It?
Obviously, the lemon battery isn’t very powerful. If you wanted to power an average 5.5 watt cell phone, you would need around 27,000 lemon batteries!
However, the same principle of the lemon battery was used by Alessandro Volta in 1800 to make a “voltaic pile” using copper and zinc disks separated by cardboard and immersed in saltwater. The output voltage of the original was about 5.5 volts, and he eventually built bigger ones that were more powerful.
British chemist Humphrey Davy used voltaic piles to experiment on gasses, such as laughing gas, and identify new elements.
Today, similar wet cell batteries, such as car batteries, use lead immersed in sulfuric acid. These can be very dangerous, but they can hold enough electrical power to start up a car or power your home.
By the Way…
If you’ve had fun with this experiment, there are hundreds more you and your child can do together to learn even more about electricity. Two classic books that go into better detail about how electricity works are:
- Getting Started in Electronicsby Forest M. Mims III
- 100 Amazing Make-It-Yourself Science Fair Projects by Glen Vecchione.
Do you have any science experiments you loved as a kid that you want to share with your fellow parents? Tell us about them in the comment section!
In Part 2, we'll create a resistor with pencil graphite in an installment called "Ohm on the Range."